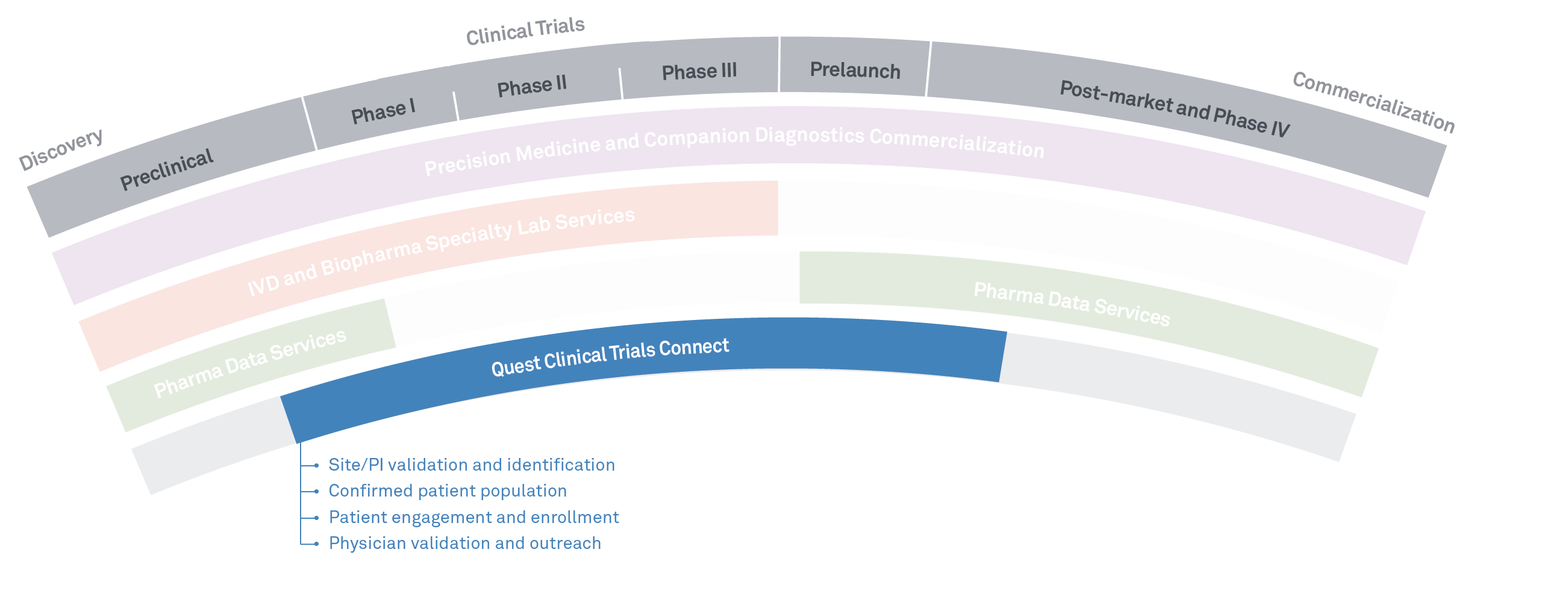
Quest Clinical Trials Connect
Using insights to make clinical trials connections

Changing the face of clinical trials
Quest Clinical Trials Connect brings patient, physician, investigator, and other third-party data together to evaluate eligibility, enroll patients, and stay continually engaged through the clinical trial process. We connect patients and physicians to clinical trial sponsors and clinical research organizations (CROs) to help drive more effective and efficient clinical trial management. Starting with powerful relationships in healthcare, Quest is building a complete, end-to-end integrated platform that’s scalable and secure.
Efficient patient and physician recruitment and site validation are available today through Quest Clinical Trials Connect. This complete solution is designed to grow with evolving demand, leading to a broad solution for cohort profiling, patient-initiated pre-screening, and virtual trials.
Targeted, complete solutions for site identification, patient enrollment, and physician outreach
Solutions for every stage of drug development
The ‘Quest’ for an Effective Partner in Pharma and Biotech
How Quest Diagnostics can be an effective partner for pharma and biotech firms.

Quest Clinical Trials Connect delivers:
- A time-saving process to identify and recruit patients for trials
- Efficient search and recruitment that can help deliver better results and better-qualified patients
- New ways to create and service virtual trials that look beyond the traditional challenges of fixed trial sites and capacity issues
- A unique data set, with 60+ billion test results covering 313+ million patient lives, and drawing from a relationship with half of US physicians and hospitals
Learn more about a patient-centered collaboration to swiftly migrate to decentralized clinical trials

End-to-end solution
Quest Clinical Trials Connect is a multiphase program designed to provide a complete, end-to-end solution including cohort analysis, trial design scenario planning, patient recruitment execution and analytics, and patient engagement. Supported with personalized management dashboards, Quest Clinical Trials Connect includes reporting tools to help measure and deliver value and impact.

Clinical Trials Connect case study
Improving clinical trial enrollment to accelerate the time to market for new therapeutics.
New video available—“Improving the Research Experience for Patients through Omnichannel Engagement and the Decentralized Trial Method,” presented by Parag More, Executive Director of Quest Healthcare Analytics Solutions
Service offerings focused around trial logistics and recruitment
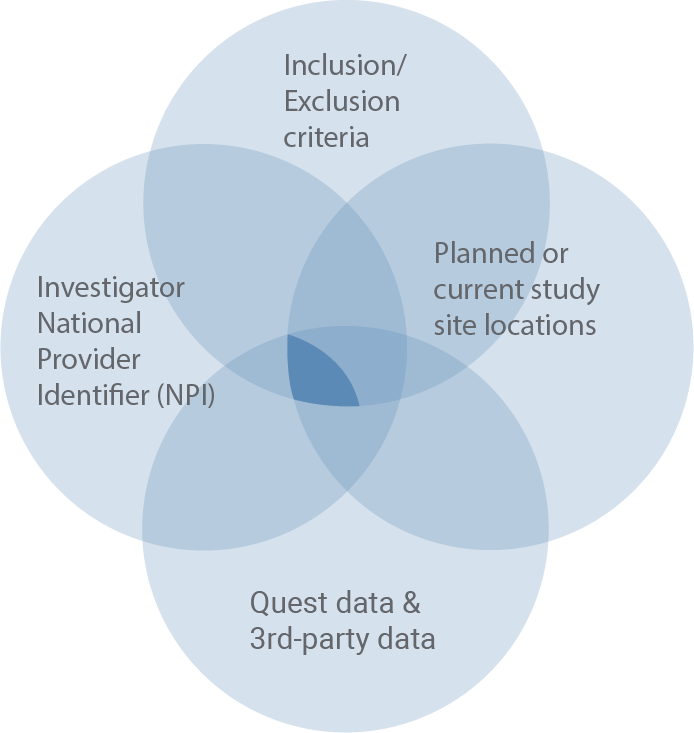
Site/Investigator validation and identification
Using the inclusion/exclusion criteria set, we can validate matched potential patient volumes to sites and investigators and help identify additional physicians with concentrations of potential patients.
Deliverables
- Validated site list
- Validated investigators
- Confirmed patient population
- Enhanced connection with physician community
Benefits
- Locates the best clinical trial sites for optimal outreach to patients of interest
- Identifies additional physicians as investigators or patient recruitment sources
- Builds confidence in selective sites/investigators
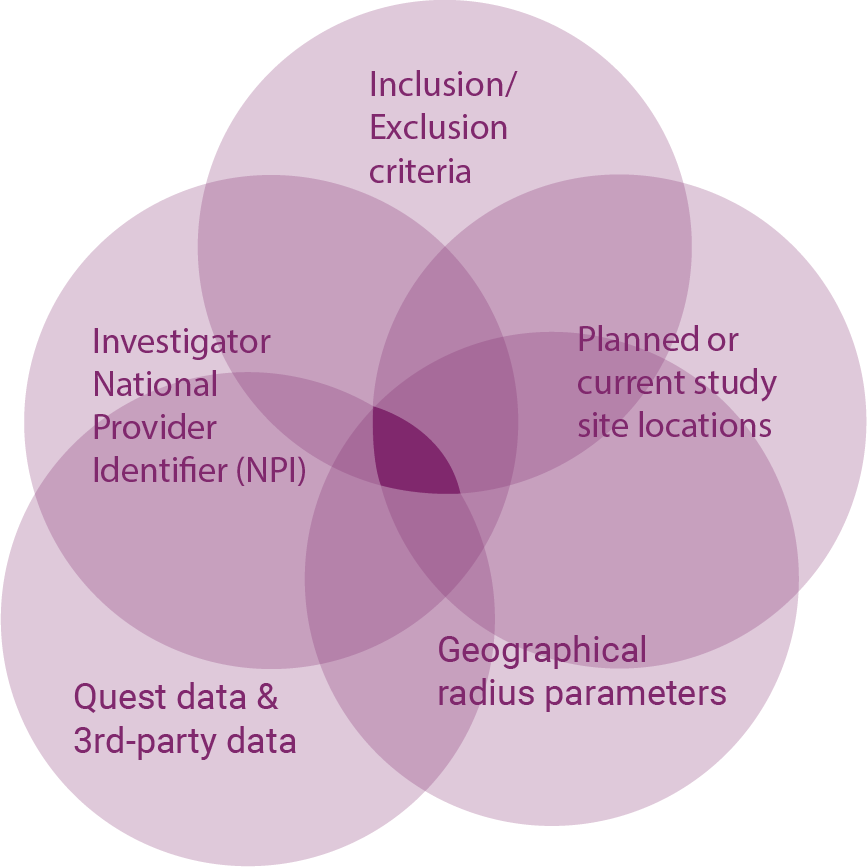
Direct patient screening
We’re bringing patients and physicians together to change the face of clinical trials, while supporting them with academic studies and leading laboratory and specimen collection services.
Deliverables
- Enrolled patients
Benefits
- Personalizes outreach for a more effective recruitment program
- Increases prescreening efficiency to lower screening burden and early attrition
- Engages patients most likely to participate through study completion
- Promotes personalized care recommendations
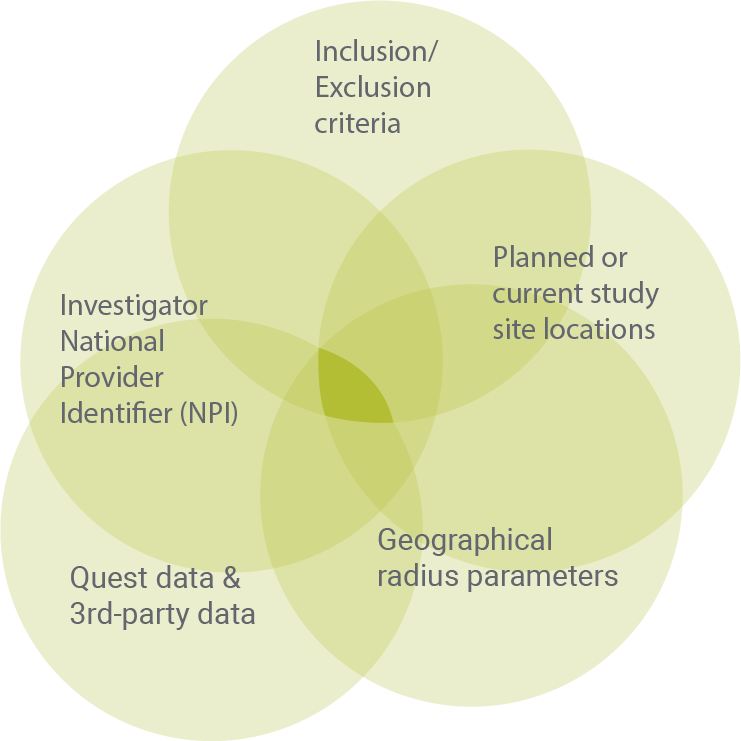
Physician outreach
We identify physicians and their patients who might qualify for clinical trials, and inform them of the opportunity.
Deliverables
- Validated site lists
- Direct access to physicians with pre-identified patients who have not opted out
- Informed and connected physician communities
Benefits
- Locates the best clinical trial sites for optimal outreach to patients of interest
- Identifies additional physicians as investigators or patient recruitment sources
- Builds confidence in selective sites/investigators
The platform
Quest Clinical Trials Connect is a secure, end-to-end, cloud-based portal with personalized access.
This secure, scalable portal cultivates and tracks patients who have opted into participation with the platform and their physicians, making the data and analytics actionable in compliance with HIPAA.
- Scalable architecture that flexes with trial complexity, geographic reach, evolutional functionality, and interoperability
- A secure, cloud-based solution that grows infrastructure and cost with evolving demand
- A collaborative approach to source subject matter expertise, platform algorithms, and data
- Machine learning measurement and analysis that builds an increasingly effective solution
- A solution engineered to measure and deliver value and impact, as defined by the sponsor
Quest Data Insights Platform™
Quest Diagnostics now offers a self-service search platform with immediate access to de-identified, cleansed, ready-to-query 60+ billion laboratory data results.
- Easily integrated with third-party sources, such as claims and prescription data
- Group patient counts by providers to quickly identify new arenas for drug development
- Identify providers with patients who meet study criteria for a drug’s clinical trials
- Understand disease prevalence and identify or expand drug development within your desired patient population inside a specific geographic location
- Identify experienced principal investigators with patients whose profiles meet the criteria for your clinical trials

“Site validation, patient recruitment, and physician recruitment solutions are only the first step in our goal of bringing clinical trials forward as a new avenue of treatment…”
Unlocking the future of patient-centered trials
Site validation, patient recruitment, and physician recruitment solutions are only the first step in our goal of bringing clinical trials forward as a new avenue of treatment to improve the health of patients. We are reimagining clinical trial design so that in the future we can incorporate:
- Direct-to-patient trials that can leverage in-home, self-administered testing and mobile care teams, who can administer therapeutics, collect samples, and capture biometric data
- Quest Patient Service Centers as potential screening sites and interim checkpoints
- Virtual Principal Investigators (PIs) or sub-PIs
Our ongoing advancements in patient-centered recruitment include:
- Enriched data sets of health conditions, physicians, and site identification
- Advanced cohort analytics
- Enrollment and retention, and experience-based outreach
Watch the webinar: Designing And Executing Decentralized Clinical Trials Using Real World Data
Put Quest Pharma Solutions to work for you